Online Study Guide
Instructions
1) You
do not need to type in your name in the space in the top-left area above. The results of this study
guide will not be sent to your teacher.
2) Carefully read each question and all the answer
choices.
3) Select the correct letter from the drop-down feature to the left of each
question.
4) When you are finished, click the “Check Your Work” button in the
bottom-left corner of the page.
5) The software will grade your work, and then it will show
you the results. Note that the correct answer will be displayed beneath the questions.
6)
Keep doing the study guide over until you earn a 100 on it.
|
|
|
1.
|
BASE
QUESTION
Acids
and bases have different properties.
As opposed to an acid, a base is most likely
to?
a. | react with limestone and
produce carbon dioxide. | b. | have a sour taste. | c. | feel slippery when mixed with
water |
|
|
|
2.
|
BASE
QUESTION
The table below lists properties of four
different pure samples.
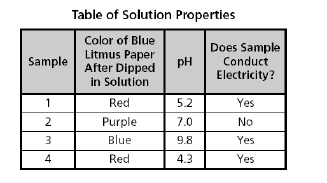 Based on the table, which sample is a
base?
a. | Sample 3 | b. | Sample 2 | c. | Sample
1 |
|
|
|
3.
|
BASE
QUESTION
Your uncle has a bottle of oven cleaner under his kitchen sink. It has
a strong odor, and the label on the bottle says it is a strong base.
What is the most likely
pH of this cleaner?
a. | pH of 12 | b. | pH of 7 | c. | pH of
2 |
|
|
|
4.
|
BASE
QUESTION
Acids and bases have unique properties that can be used to identify
them.
Which properties most likely indicate that a substance is a
base?
a. | releases hydrogen gas and reacts with
metals | b. | has a pH below seven and corrodes a
nail | c. | turns red litmus blue and conducts an electric
current |
|
|
|
5.
|
BASE
QUESTION
An indicator such as litmus paper is any
chemical that has a change in color when added to either an acid or a base.
Which of these
common household substances would turn red litmus paper blue?
a. | coffee | b. | vinegar | c. | borax
solution |
|
|
|
6.
|
BASE
QUESTION
Think about the key properties that bases have.
An unknown
substance will be classified as a base if the substance...?
a. | releases hydroxide ions in
water. | b. | turns blue litmus paper red | c. | has a pH of 3.0. |
|
|
|
7.
|
BASE
QUESTION
Acid-Base
Indicators | Indicator | Color in Acid | Color in Base | | Litmus | Red | Blue | | Bromthymol
blue | Yellow | Blue | | Methyl red | Red | Yellow | | Phenolphtalein | Colorless | Pink | | | |
Johnny added two drops of Phenolphtalein to a solution of water and an unknown
substance. The solution gradually turned pink.
This color change indicates a pH in the _______
range (acidic or basic) and an excess of ___________ (hydronium or hydroxide) ions in
the solution.
a. | basic, hydronium ions | b. | acidic, hydronium ions | c. | basic, hydroxide
ions |
|
|
|
8.
|
BASE
QUESTION
Where in this Venn diagram would we add
these phrases “Used in soap & is slippery?”
a. | With letter C | b. | With letter D | c. | With letter
A |
|
|
|
9.
|
BASE
QUESTION
Bases have unique properties and uses that can be used to identify
them.
Which properties shown below most likely indicate that a substance is a
base?
a. | turns blue litmus paper red and conducts an electric
current | b. | used in oven cleaners and in products that unclog drains, pH range 7.1 to
14 | c. | has a pH range below seven, is used to make paper,
paint, and plastic |
|
|
|
10.
|
BASE
QUESTION
Acid-Base Indicators | Indicator | Color in Acid | Color in Base | | Litmus | Red | Blue | | Bromthymol blue | Yellow | Blue | | Methyl red | Red | Yellow | | Phenolphtalein | Colorless | Pink | | | |
Jasmine added 30 mL of Methyl Red to a solution of water and and an unknown
substance. Then the teacher told her that the solution was a very strong base.
What color did
the solution become after she added the Methyl Red to it, and what was the likely pH
level?
a. | Blue, ph of 12 | b. | Yellow, pH of 2 | c. | Yellow, pH of
13 |
|