Online Assessment
Instructions
1) Type in your first and last name in the
“Name” box in the top-left corner.
2) Next type in your teacher’s name in
the “ID” box.
3) Then type in your school’s full name in the
“Email” box.
4) Select the best answer for each question.
5) When you
are finished click the “Grade and Submit” button.
6) The grade will be emailed
to your teacher.
|
|
|
1.
|
NOTE: If your teacher’s last
name is Elliott, then you are doing the wrong assessment.
---This assessment is only for
students of other teachers.
---If your teacher is Mr. Elliott, then please go back to the
website and click on the first assessment link instead. Thanks!
ACID QUESTION
Question with
Two-Parts
(A) What will begin to happen when the lake water reaches a pH level of
6.0?
(B) Would the water at that pH level be considered a strong acid or a weak
acid?
a. | (A) rainbow trout begin to die
(B) weakly acidic | c. | (A)
Frogs will begin to mate
(B) strongly
acidic | b. | (A) All organisms will thrive
(B) strongly acidic | d. | (A) All fish
will be killed
(B) weakly
acidic |
|
|
|
2.
|
ACID
QUESTION
You find an unlabeled bottle of clear liquid in the refrigerator. You
pour a small amount of this liquid into a cup and taste it. It has a distinct tangy/sour flavor.
Two-Part Question
A) What is the pH of this liquid?
B) Is this liquid acidic or
basic?
a. | A) 14
B) It is acidic | b. | A) above 7
B) It is
basic | c. | A) below 7
B) It is acidic | d. | A) 7
B) It is
neutral |
|
|
|
3.
|
ACID
QUESTION
Which of these common household
substances would turn blue litmus paper red, and why?
a. | tomato juice, it has acid in it | c. | blood, it has acid in it | b. | milk of magnesia,
it is a base | d. | tomato juice, it has a base in it |
|
|
|
4.
|
ACID
QUESTION
In your refrigerator at home you find a bottle of lemon juice. You
remember that your teacher told you that lemons have a pH around 2.0.
Which choice below best
describes this lemon juice?
a. | basic with a sour taste | c. | basic with bitter
taste | b. | acidic with a sour taste | d. | acidic with a bitter taste |
|
|
|
5.
|
ACID
QUESTION
Acid-Base
Indicators | Indicator | Color in Acid | Color in Base | | Litmus | Red | Blue | | Bromthymol
blue | Yellow | Blue | | Methyl red | Red | Yellow | | Phenolphtalein | Colorless | Pink | | | |
Sylvia added five drops of bromthymol blue in a beaker containing a solution
of water and carbon dioxide. The solution gradually turned yellow.
Question
This color change indicates a pH in the _______ range (acidic or
basic) and an excess of ___________ in the solution (hyrdonium or hyrdroxide).
a. | basic, hydronium ions | c. | basic, hydroxide ions | b. | acidic, hydroxide
ions | d. | acidic, hydronium
ions |
|
|
|
6.
|
ACID
QUESTION
An unknown substance will be classified as an acid if the
substance...?
a. | releases hydroxide ions in water. | c. | turns red litmus paper blue. | b. | is slippery to the touch. | d. | has a pH of
3.0. |
|
|
|
7.
|
ACID
QUESTION
The activity of an enzyme at different pH
levels is shown in the graph.
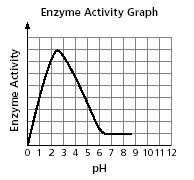 Which table best represents the data in the
graph?
|
|
|
8.
|
ACID
QUESTION
Litmus paper is made from water-soluble
dyes which are extracted from lichens. This paper is used as an acid-base indicator.
Which of these household substances would turn blue litmus
paper red?
a. | toothpaste | b. | ammonia | c. | soap | d. | vinegar |
|
|
|
9.
|
ACID
QUESTION
Acid and Base: Venn Diagram
Where in this Venn diagram would we add this phrase “Increases
hydronium ions?”
a. | With letter C | c. | With letter B | b. | With letter A | d. | With letter D |
|
|
|
10.
|
ACID
QUESTION
Based on this table, which two samples are
acids?
a. | Samples 1 and 4 | c. | Samples 2 and 3 | b. | Samples 4 and 3 | d. | Samples 1 and 3 |
|