Online Study Guide
Instructions
1) You
do not need to type in your name in the space in the top-left area above. The results of this study
guide will not be sent to your teacher.
2) Carefully read each question and all the answer
choices.
3) Select the correct letter from the drop-down feature to the left of each
question.
4) When you are finished, click the “Check Your Work” button in the
bottom-left corner of the page.
5) The software will grade your work, and then it will show
you the results. Note that the correct answer will be displayed beneath the questions.
6)
Keep doing the study guide over until you earn a 100 on it.
|
|
|
1.
|
Table salt (NaCl) is the product of a chemical
reaction. | | |
Below is a description of this chemical reaction.
Two atoms of Sodium
plus two atoms of Chlorine yields one Sodium Chloride molecule
Which of the following choices is the product of this
reaction?a. | Sodium Chloride | b. | Chlorine | c. | Sodium |
|
|
|
2.
|
Iron and oxygen chemically react to form a compound
called iron oxide. This is commonly known as rust. The chemical equation for this reaction is shown
below.
Identify the reactants in this chemical
equation.
a. | O (Oxygen) and Fe (Iron) | b. | Rust and
Oxygen | c. | 2FeO3 (Iron
Oxide) |
|
|
|
3.
|
The equation below describes the
chemical reaction that forms table salt (NaCl).
Two atoms of Sodium + Two
atoms of Chlorine  One Sodium Chloride molecule a. | NaCl | b. | Chlorine | c. | Sodium
Chloride |
|
|
|
4.
|
The equation below illustrates the reaction that
occurs in chloroplasts during photosynthesis.
Which is a product of
photosynthesis?
a. | oxygen | b. | water | c. | carbon
dioxide |
|
|
|
5.
|
Rocket ships use powerful chemical reactions to
blasts off as shown the following equation.
10Al + 6NH4ClO4  4Al2O3 + 12H2O + 3N2
4Al2O3 + 12H2O + 3N2
Identify the
reactants from this chemical equation.
a. | Al and NH4ClO4
| b. | N and Al2O3 | c. | H2O and N |
|
|
|
6.
|
Have an upset stomach? You can take Alka
Seltzer tablets. Drop two tablets in water and you get a chemical
reaction.
Two-Part Question
A) How many reactants does this chemical
equation contain?
B) Identify two of the products.
a. | A. Three products
B. Sodium (Na) and Carbon (C) | b. | A. Two
reactants
B. Water (H2O) & Carbon Dioxide
(CO2) | c. | A. Two reactants
B. Nitrogen (N) and Water
(H2O) |
|
|
|
7.
|
The formula below
represents a chemical reaction.
Sn (Tin) + 2H2O (Water) è
SnO2 (Tin Dioxide) + 2H2 (Hydrogen)
Which substance is a product of this reaction?a. | SnO2 (Tin Dioxide) | b. | H2O (Water) | c. | NaCl (sodium
chloride) |
|
|
|
8.
|
A chemical equation is shown
below.
C6H12O6 +
6O2  6CO2 +
6H2O 6CO2 +
6H2O
What are the products of the reaction?a. | C6H12O6 and
CO2 | b. | CO2
and H2O | c. | O2 and
C6H12O6 |
|
|
|
9.
|
The equation below represents a chemical reaction that releases
energy.
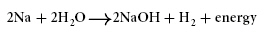 Which part of this equation is a reactant?
|
|
|
10.
|
The Space Shuttle used solid rocket propellant
called APCP. Burning APCP results in a very powerful chemical reaction.
Two-Part
Question
A) How many products does this chemical equation contain?
B) Identify two of
the products.
a. | A. Four products
B. Nitrogen (N) and Water (H2O) | b. | A. Two
products
B. Water (H2O) and Nitrogen (N) | c. | A. Six Products
B. Aluminum (Al) and
Chlorine (Cl) |
|