Multiple Choice
Identify the choice
that best completes the statement or answers the question.
|
|
|
1.
|
All three of the elements shown below are in period
four.
How
many electron shells does each atom of calcium contains, and what is the atomic mass of titanium?
a. | All elements in period 4 have 4 electron shells.
Titanium’s atomic mass is 47.9 | b. | All elements in
the 4th period have 6 electron shells. Titanium’s atomic mass is
47.9 | c. | All elements in period 4 have 4 electron shells.
Titanium’s atomic mass is 22 |
|
|
|
2.
|
A periodic table of the elements is shown below.
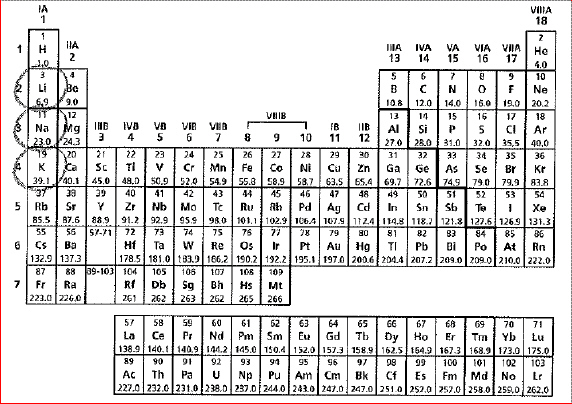 The circled elements share which characteristic? The circled elements share which characteristic?
a. | They have the same number of electrons. | b. | They rarely form
chemical bonds. | c. | They are alkali metals |
|
|
|
3.
|
In the periodic table shown below, some elements
are shaded.
Which shaded element shown above has the
least amount of mass and the least amount of protons?
a. | helium (He) | b. | neon
(Ne) | c. | argon (Ar) |
|
|
|
4.
|
In the periodic table shown below, some elements
are shaded.
Based on its position in the periodic
table, which element is the heaviest?
a. | helium (He) | b. | krypton (Kr) | c. | neon
(Ne) |
|
|
|
5.
|
A periodic table of
the elements is shown below.
Based on their locations on the periodic table, which two elements share the
most similar chemical
properties?
a. | Be and
Ba | b. | S and
Sn | c. | H and
I |
|
|
|
6.
|
All three of these elements are in the second
period.
12.0
C
Carbon
6 | 14.0
N
Nitrogen
7 | 15.9
O
Oxygen
8 | | | |
Two-Part Question
How many electron shells does each atom of
nitrogen contain, and what is the atomic number of carbon?
a. | They all have 4 electron shells. Carbon’s atomic
number is 6. | b. | Nitrogen has 7
electron shells. Carbon’s atomic mass is 12.0 | c. | They all have 2 electron shells. Carbon’s atomic number is
6. |
|
|
|
7.
|
Examine the nobel gases shown below in group
18.
Two-Part Question
A) What is
the number of valence electrons for Neon (Ne), Argon (Ar), and Krypton (Kr)?
B) Which of these
elements only has two protons?
a. | 8 valence electrons, and Helium has two
protons. | b. | 6 valence electrons, and Argon has two
protons | c. | 8 valence electrons, and Neon has two
protons |
|
|
|
8.
|
Examine the three shaded elements shown below in
the 4th period.
A property that elements Fe, Co and Ni have in common is that they are
all...?
a. | transition
metals. | b. | chemically
inert. | c. | poor electrical
conductors. |
|
|
|
9.
|
Use the Periodic Table below to answer the
following question.
How many protons does the element Neon (Ne) have and how many electron
shells does it have?
a. | 2 protons, and 8 electron
shells | b. | 10 protons, and 2 electron
shells | c. | 10 protons, and 18 electron
shells |
|
|
|
10.
|
The periodic table of elements is shown
below.
Which statement best describes the
reactivity of the shaded elements nitrogen (N), oxygen (O), and fluorine (F)?
a. | Oxygen is the most reactive of all the elements
listed. | b. | The nonmetals decrease in reactivity from right to left
in the same period. | c. | The nonmetals
increase in reactivity from right to left in the same period. |
|