Online Study Guide
Instructions
1) You
do not need to type in your name in the space in the top-left area above. The results of this study
guide will not be sent to your teacher.
2) Carefully read each question and all the answer
choices.
3) Select the correct letter from the drop-down feature to the left of each
question.
4) When you are finished, click the “Check Your Work” button in the
bottom-left corner of the page.
5) The software will grade your work, and then it will show
you the results. Note that the correct answer will be displayed beneath the questions.
6)
Keep doing the study guide over until you earn a 100 on it.
|
|
|
1.
|
What is the atomic mass of an atom of
rubidium, and how many neutrons does each rubidium atom have? a. | Rubidium has 49 neutrons, and the atomic mass is
85.5 | b. | Rubidium has 37 neutrons, and the atomic mass is
85.5 | c. | Rubidium has 86 neutrons, and the atomic mass is
37 |
|
|
|
2.
|
A group from the periodic table of elements is
shown below.
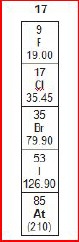
What
is the atomic number of fluorine (F)?
|
|
|
3.
|
The diagram
below shows part of the periodic table of the elements.
Two-Part Question:
Which element from period 4 has the highest
atomic mass, and which element from group 17 has the lowest atomic
number?
a. | Highest atomic mass-Kr
(Krypton)
Lowest atomic
number-F (Flourine) | b. | High atomic mass-Fe (Iron)
Low atomic number- Ni (Nickle) | c. | High atomic mass-K
(Potassium)
Low atomic
number- Ne (Neon) |
|
|
|
4.
|
How many neutrons does each boron atom contain, and what is Boron’s
atomic number? a. | Boron has 16 neutrons, and the atomic number is
5. | b. | Boron has 10.8 neutrons, and the atomic number is
15.8 | c. | Boron has 6 neutrons, and the atomic number is
5. |
|
|
|
5.
|
How many total protons does each nickel atom contain, and what is the atomic
mass of Cobalt? a. | Nickel has 28 protons, and the atomic mass of Cobalt is
58.9. | b. | Cobalt has 28 electrons, and it is odd that
cobalt’s atomic mass is less than nickel’s mass. | c. | Nickel has 27 electrons, and the atomic mass of Nickel is
58.7 |
|
|
|
6.
|
How
many protons, electrons and neutrons does each titanium atom contain? a. | Titanium atoms have 22 protons, 22 electrons and 69.9
neutrons. | b. | Titanium atoms have 22 protons, 22 electrons and 26
neutrons. | c. | Titanium atoms have 22 protons, 22 electrons and 47.9
neutrons. |
|
|
|
7.
|
How many electrons and neutrons does each
neon atom contain? a. | Neon atoms have 20 electrons and 10 neutrons.
| b. | Neon atoms have 30.2 electrons and 20 neutrons.
| c. | Neon atoms have 10 electrons and 10 neutrons.
|
|
|
|
8.
|
A group from the periodic table of elements is
shown below.
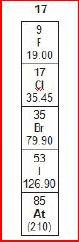
What
is the atomic mass of Bromine and the atomic number of Iodine?a. | 35 and 79.90 | b. | 79.90 and 53 | c. | 9 and
19.00 |
|
|
|
9.
|
Contrast atomic number and atomic
mass. a. | Atomic number is the number of neutrons in one
atom of an element.
Atomic mass is the average amount
of matter in one atom of an element | b. | Atomic number
is the number of protons in one atom of an element.
Atomic mass is the average amount of matter in one atom of an
element | c. | Atomic mass is the number of protons in one atom
of an element.
Atomic number is the average amount of
matter in one atom of an element. |
|
|
|
10.
|
The diagram
below shows part of the periodic table of the elements.
Which element
from Period 2 has the lowest atomic mass?
|