Online Assessment
Instructions
1) Type in your first and last name in the
“Name” box in the top-left corner.
2) Next type in your teacher’s name in
the “ID” box.
3) Then type in your school’sfull name in the
“Email” box.
4) Select the best answer for each question.
5) When you
are finished click the “Grade and Submit” button.
6) The grade will be emailed
to your teacher.
|
|
|
1.
|
NOTE: If your teacher’s last name is Elliott, then you are
doing the wrong assessment.
---This assessment is only for students of other teachers.
---If your teacher is Mr. Elliott, then please go back to the website and click on the first
assessment link instead. Thanks!
The periodic table of elements is shown below.
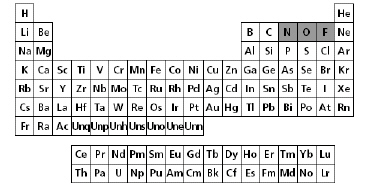
a. | The nonmetals increase in reactivity from right to left
in the same period. | b. | Oxygen is the most
reactive of all the elements listed. | c. | Fluorine is the
least reactive of all the shaded elements. | d. | The nonmetals
decrease in reactivity from right to left in the same period. |
|
|
|
2.
|
Look at the diagram below of one atom of
boron (B).
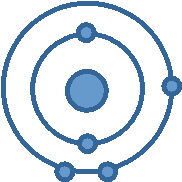
Two-Part Question
a) Based on the number of valence electrons,
what group number is boron found in?
b) Based on the number of electons shells, what period
number is boron found in? a. | Group 3, Period 3 | c. | Group
13, Period 5 | b. | Group 2, Period
13 | d. | Group 13, Period 2 |
|
|
|
3.
|
The periodic table of elements is shown
below.
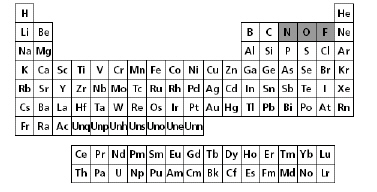
Which statement best describes the reactivity of the shaded elements nitrogen
(N), oxygen (O), and fluorine (F)?a. | The nonmetals increase in reactivity from left to right
in the same period. | b. | Oxygen is the most
reactive of all the elements listed. | c. | The nonmetals
increase in reactivity from right to left in the same period. | d. | Fluorine is the least reactive of all the shaded
elements. |
|
|
|
4.
|
A periodic table of the elements is
shown below.
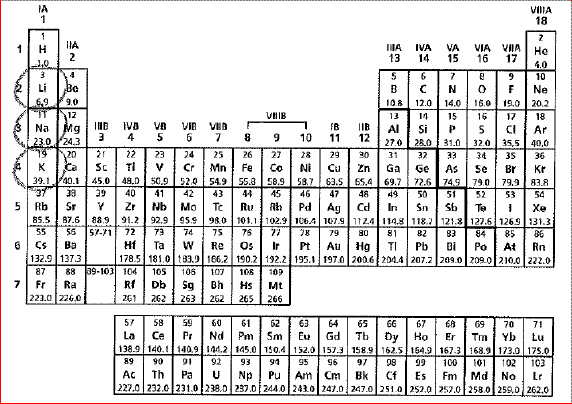 The circled elements share which
characteristic? The circled elements share which
characteristic?a. | They are nonmetals | b. | They rarely form chemical
bonds. | c. | They are alkali metals | d. | They have the same number of
electrons. |
|
|
|
5.
|
A periodic table of the elements is shown
below.
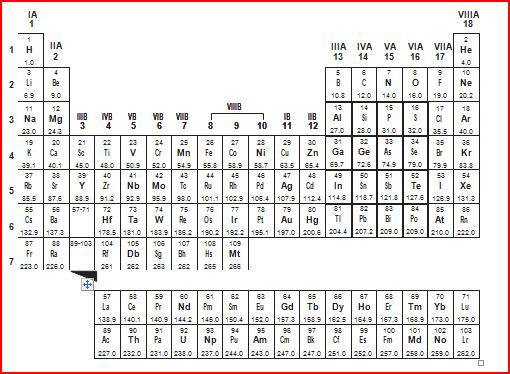
Based on their locations on the periodic table, which two elements share the
most similar chemical
properties? a. | He and
Li | b. | H and
Kn | c. | Ne and
Xe | d. | K and
Ca |
|
|
|
6.
|
All three of these elements are in the second
period.
12.0
C
Carbon
6 | 14.0
N
Nitrogen
7 | 15.9
O
Oxygen
8 | | | |
Two-Part Question
How many electron shells does each
atom of nitrogen contain, and what is the atomic number of carbon?
a. | Nitrogen has 7 electron shells. Carbon’s atomic
mass is 12.0 | b. | They all have 2
electron shells. Carbon’s atomic number is 6. | c. | They all have 4 electron shells. Carbon’s atomic number is
6. | d. | They all have 2 electron shells. Nitrogen’s atomic
number is 6. |
|
|
|
7.
|
Look at the elements in the shaded column above (helium (He), neon (Ne), argon
(Ar, and krypton (Kr). Determine what group number they are in, and determine how many electron
shells krypton has.a. | Group 4, 6 electron shells | c. | Group 18, 4 electron shells | b. | Group 18, 7 electron shells | d. | Group 7, 8
electron shells |
|
|
|
8.
|
A
periodic table of the elements is shown below.
What do all three of the shaded elements shown
above (Fe, Co and Ni) have in common? a. | They are all metals with 4
valence electrons. | b. | They are all halogens with 4 electron shells. | c. | They are all poor electrical
conductors. | d. | They are all metals with 4 electron
shells. |
|
|
|
9.
|
A property that
elements Fe, Co and Ni have in common is that they are all...? a. | chemically
inert. | c. | poor electrical
conductors. | b. | halogens. | d. | transition metals. |
|
|
|
10.
|
Two-Part Question
Which elements of the periodic table are shaded
above and what is one key property of these elements? a. | Metals, and they are good electrical
conductors. | b. | Metalloids, and
they do not conduct electricity. | c. | Lanthanides and
Actinides. They are all non-reactive noble gases | d. | Nonmetals, and they are good electrical
conductors. |
|