Online Assessment
Instructions
1) Type in your first and last name in the
“Name” box in the top-left corner.
2) Next type in your teacher’s name in
the “ID” box.
3) Then type in your school’s name in the
“Email” box.
4) Select the best answer for each question.
5) When you
are finished click the “Grade and Submit” button.
6) The grade will be emailed
to your teacher.
|
|
|
1.
|
NOTE: If your teacher’s last
name is Elliott, then you are doing the wrong assessment.
---This assessment is only for
students of other teachers.
---If your teacher is Mr. Elliott, then please go back to the
website and click on the first assessment link instead. Thanks!
If some rain water happens to seep down into a crevice in a rock and it
freezes, the volume of the ice will increase and it can crack the rock as it expands. This is an
example of weathering. Which of the following statements
most accurately describes this type of weathering?
a. | The rock remained unchanged during this weathering
process. | b. | This is a physical change since heat was given off and
gas was released. | c. | The rock
physically changed as ice expanded inside the crack. | d. | A chemical change
occurred as the ice cracked the rock. |
|
|
|
2.
|
Which statement best describes a physical
change?
a. | A process separates water into hydrogen and
oxygen. | b. | Wood burning on a camp fire releases gases into the
air. | c. | An empty Coke can is crushed
flat. | d. | A copper penny exposed to the air forms rust (copper
oxide) |
|
|
|
3.
|
When air is heated, it expands and rises. Hot-air
balloon pilots use this phenomena to their advantage. These pilots use a gas-flame blower to increase
the temperature of the air inside the balloon making the balloon rise.
This
description of hot-air balloon operations includes a physical change example and a chemical change
example.
Select the choice below that correctly identifies
both of these changes.
a. | The heated air is a chemical change. The burning fuel is
a physical change. | b. | The balloon rising
is a chemical change due to overcoming the force of gravity. | c. | The wind blowing the balloon is a physical change. The flame is a chemical
change. | d. | The burning fuel is a chemical change. The heated air is
a physical change. |
|
|
|
4.
|
Which of the following is an example of a physical change but NOT a
chemical
change?
a. | A child forgets to put the jug of milk back in the refrigerator, and it turns
sour. | b. | A wood log burns on a camp fire. | c. | An old car rusts away out in a grassy
field. | d. | A man boils water to make hot tea. |
|
|
|
5.
|
A group of students observed a glass of water
inside a vacuum chamber. The vacuum chamber was attached to a pump that sucked out all of the air
from the chamber. When the air was removed, the water began to boil at room temperature.
Which of the following statements BEST explains what happened in the scenario
described above?
a. | The water underwent a physical change and turned into
vapor when air pressure was removed . | b. | Water is naturally
liquid and must undergo a chemical change in order to boil at room
temperature. | c. | The temperature
increased until the water boiled. This is evidence of a chemical change. | d. | When the air was removed, the water molecules could no longer float. Therefore
the liquid began to boil. |
|
|
|
6.
|
During phase changes, the appearance of a substance
is changed, but there is no change at the atomic level.
Which of the following is an
example of a physical change (a change of phase) but NOT a chemical change?
a. | A tree stores energy from the Sun in its fruit. | b. | A penny lost in the
grass slowly changes color. | c. | A water pipe freezes and cracks on a cold
night. | d. | A log gives off heat and light as it burns. |
|
|
|
7.
|
A student in science class combined two liquids.
The temperature increased, a foul smell was given off, and a solid substance was formed.
The student could best classify this reaction as a...?
a. | physical change. | c. | mass
change. | b. | weight change. | d. | chemical
change. |
|
|
|
8.
|
A green powder (a solid) and clear water (a liquid)
were combined together in a beaker.
After combining these two items, observations were recorded.
Which of the following observations indicates that a chemical change
occurred?
a. | The combination turned a green
color. | b. | Nothing special was observed. The powder merely sank to
the bottom of the liquid. | c. | The powdery
substance dissolved into the clear liquid. | d. | A gas was
released, a popping noise was heard, and the temperature
increased. |
|
|
|
9.
|
Copper carbonate being heated in a test
tube is shown in the diagram below.
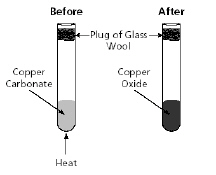 Which statement best describes what happened in
this experiment?a. | Copper oxide decomposed when heated, causing a chemical
change. | b. | Copper oxide reacted with the glass wool, causing a
physical change. | c. | Copper carbonate
reacted with the glass wool, causing a physical change. | d. | Copper carbonate decomposed
when heated, causing a
chemical change. |
|
|
|
10.
|
Two students combined a solid with a liquid in a container. They
observed a flash, and they saw and smelled gas being released. They also noticed that the container
grew hot to the touch.
How can they be certain that they observed a chemical change instead of
just a physical change.
a. | The saw it flash, felt the
temperature increase, and observed gas being released | b. | The can be certain because the liquid changed into
a gas (change of state) | c. | The know this was a chemical change because it changed
color. | d. | This was definitely a chemical reaction because the solid dissolved in the
liquid. |
|