SPI 0807.9.7 (Density)
|
|
|
1.
|
Your science teacher conducts a density column demonstration using the following
3 liquids. | Designation | Substance | Density | | Liquid 01 | Water
| 1.0 g/mL | | Liquid
02 | Liquid Mercury | 13.6 g/mL | | Liquid 03 | Turpentine | 0.8 g/mL | | | |
He pours each liquid into a graduated cylinder. Which liquid would
occupy level A?
|
|
|
2.
|
| Sample | A | B | C | D | | Volume | 100
cc | 100 cc | 100 cc | 100
cc | | Mass | 100 g | 80
g | 108 g | 90 g | | Density | 1
g/cc | 0.8 g/cc | 1.08 g/cc | 1.2
g/cc | | | | | |
Which density value shown above is NOT
correct? a. | Sample D | b. | Sample C | c. | Sample
A |
|
|
|
3.
|
The equation for density is shown below.
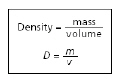 Which block has the GREATEST density?
|
|
|
4.
|
The density for aluminum is
2.7g/cm3. What
would be the VOLUME of a piece of aluminium that has a mass of 13g?
HINT: Remember the density formula is density=mass divided by volume. You can rewrite this
formula like this mass=density x volume or like this volume=mass divided by
density. a. | 0.207cm3 | b. | 4.81cm3 | c. | 4.81g/cm3 |
|
|
|
5.
|
A chart listing the density of four metals is shown below.
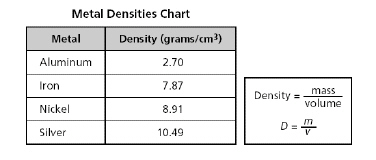
A student determines that
a metal has a mass of 810.0 grams and a volume of 90.0 cubic centimeters. Using the formula above,
this unknown metal is most likely...?a. | aluminum | b. | silver | c. | nickel |
|
|
|
6.
|
Sample | Volume | Mass | A | 100 ml | 100 g | B | 100 ml | 80 g | C | 100 ml | 108 g | D | 100 ml | 90 g | | | |
The table shows the masses of different samples of liquid. Which sample has
the LEAST density?a. | Sample A | b. | Sample B | c. | Sample
D |
|
|
|
7.
|
While doing an experiment in science class, Lenny
accidentaly drops his pencil into a graduated cylinder. The pencil has a mass of 12 grams and the
water level in the cylinder rises from 41 ml to 44 ml. Determine the density of the
pencil.
a. | 4 g/ml | b. | 50
g/ml | c. | 0.5 g/ml |
|
|
|
8.
|
The density for iron is 7.874
g/cm3. What would
be the MASS of a 25 cm3 piece of iron?
HINT: Remember the density formula is density=mass divided by volume. You can rewrite this
formula like this mass=density x volume or like this volume=mass divided by
density. a. | 196.75g | b. | 3.18g | c. | 196.85g |
|
|
|
9.
|
A student is given an object and is asked to
identify its density. The object has a volume of 3 cubic centimeters and a mass of 6
grams.
Using the formula above, what is the density of
the object?
a. | 2 grams/cubic centimeter | b. | 9 grams/cubic centimeter | c. | 3 grams/cubic
centimeter |
|
|
|
10.
|
A 5-gram sample of water occupies 5 milliliters of
space in a beaker.
Using the above formula, what is the density of
the water sample?
a. | 1 g/mL | b. | 10
g/mL | c. | 25 g/mL |
|