Online Assessment
Instructions
1) Type in your first and last name in the
“Name” box in the top-left corner.
2) Next type in your teacher’s name in
the “ID” box.
3) Then type in your school’s full name in the
“Email” box.
4) Select the best answer for each question.
5) When you
are finished click the “Grade and Submit” button.
6) The grade will be emailed
to your teacher.
|
|
|
1.
|
NOTE: This assessment is only for Mr.
Elliott’s students.
---If you are not one of Mr. Elliott’s students then please go
back to the website and click on the link for other teachers. Thanks!
A chart
listing the density of four metals is shown below.
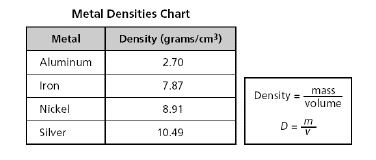
A
student determines that a metal has a mass of 12 grams and a volume of 1.52 cubic centimeters. Using
the formula above, this unknown metal is most likely...?
a. | nickel | b. | aluminum | c. | silver | d. | iron |
|
|
|
2.
|
Substance | Mass | Volume | Density | Liquid A | 13.6g | 20cm3 | 0.68g/cm3 | Copper | 17.88g | 2cm3 | ??? | Solid 01 | 80g | 50cm3 | 1.6g/cm3 | Solid 02 | 36g | 50cm3 | .72g/cm3 | | | | |
Two part
question:
(A) What is the density of copper?
(B)
Which has greater density, copper or solid 01?a. | A) 8.94 g/cm3
B) solid 01 is more dense | c. | A) 8.94 g/cm3
B) copper is more dense | b. | A) .68 g/cm3
B) liquid A is more dense | d. | A)
1.6g/cm3
B) solid 01 is more
dense |
|
|
|
3.
|
Your science teacher makes a density column using 3 of the following 5
liquids. | Designation | Substance | Density | | Liquid 01 | Honey | 1.5 g/mL | | Liquid 02 | Coca Cola | 1.1 g/mL | | Liquid 03 | Water
| 1.0 g/mL | | Liquid 04 | Motor Oil | 0.9 g/mL | | Liquid 05 | Corn Syrup | 1.4 g/mL | | | |
If he poured in the liquid from
the table with the greatest density and the liquid with the least density, which liquids would be
located a levels A and C?a. | Level A: Water
Level C: Coca Cola | c. | Level A: Honey
Level
C: Corn Syrup | b. | Level A: Honey
Level C: Motor
Oil | d. | Level A: Motor
Oil
Level C: Honey |
|
|
|
4.
|
The
density for iron is 7.874 g/cm3.
What would be the MASS of a 25 cm3 piece of
iron?
HINT: MVD (mass = volume x density)
a. | 3.18g | c. | 393.7g | b. | 196.85g | d. | 196.85g/cm3 |
|
|
|
5.
|
Sample | Volume | Mass | W | 100
ml | 100 g | X | 100
ml | 80 g | Y | 100
ml | 108 g | Z | 100
ml | 90 g | | | |
The table shows the masses of different samples of liquid.
Which sample
has the GREATEST density?a. | Sample W | b. | Sample X | c. | Sample Z | d. | Sample
Y |
|
|
|
6.
|
The density for aluminum is
2.7g/cm3.
What would the VOLUME be for a piece of
aluminium that has a mass of 13g?
HINT: VDM
volume = density divides the mass (mass divided by density).
a. | 4.81g/cm3 | c. | 4.81cm3 | b. | 35.1cm3 | d. | 0.207cm3 |
|
|
|
7.
|
| Sample | A | B | C | D | | Volume | 100 cc | 100 cc | 100 cc | 100 cc | | Mass | 100 g | 80 g | 108 g | 90 g | | Density | 1 g/cc | 0.8 g/cc | 1.08 g/cc | 1.2 g/cc | | | | | |
Which density value shown above is NOT
correct?a. | Sample D | b. | Sample B | c. | Sample A | d. | Sample
C |
|
|
|
8.
|
The equation for density is shown below.
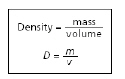 Which block has the GREATEST density?
|
|
|
9.
|
While doing an experiment in science class, Kathy
accidentaly drops her pencil earser into a graduated cylinder. The eraser has a mass of 4 grams and
the water level in the cylinder rises from 90 ml to 92 ml.
What is the density of the eraser?
a. | 2 g/ml | b. | 2
grams | c. | 4 g/ml | d. | 90
g/ml |
|
|
|
10.
|
| Sample | A | B | C | D | | Volume | 30 cc | 20
cc | 10 cc | 10 cc | | Mass | 100 g | 80 g | 108 g | 25
g | | Density | 3.5 g/cc | 4 g/cc | 10.8 g/cc | 2.5 g/cc | | | | | |
Justin and Noah made a mistake when
calculating one of the density values for one of the four samples shown above.
Which
density value shown above is NOT correct?a. | Sample
A | b. | Sample B | c. | Sample
C | d. | Sample D |
|