Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
For the diagram shown. What part of the atom does “A”
represent? 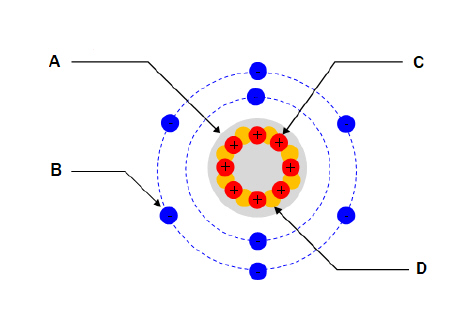 a. | Nucleus | c. | Proton | b. | Electron | d. | Neutron |
|
|
|
2.
|
For the diagram shown. What part of the atom does “B”
represent? 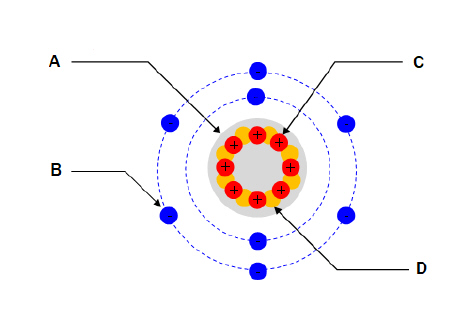 a. | Nucleus | c. | Proton | b. | Electron | d. | Neutron |
|
|
|
3.
|
For the diagram shown. What part of the atom does “C”
represent? 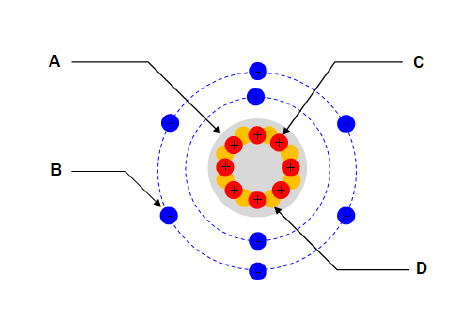 a. | Nucleus | c. | Proton | b. | Electron | d. | Neutron |
|
|
|
4.
|
For the diagram shown. What part of the atom does “D”
represent? 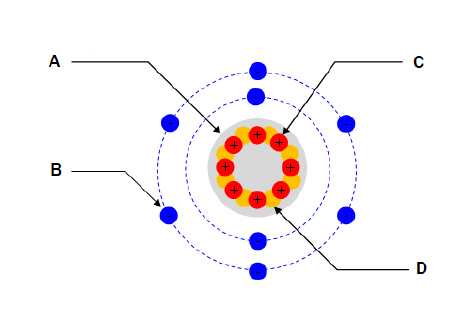 a. | Nucleus | c. | Proton | b. | Electron | d. | Neutron |
|
|
|
5.
|
For the diagram shown. What element is this? (You may need to google a
periodic table of elements) 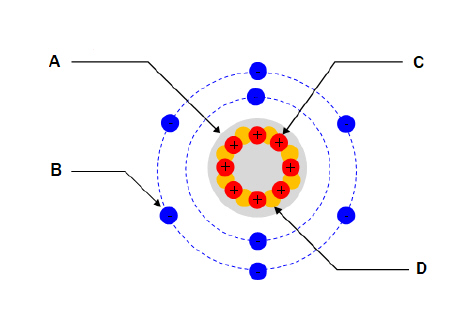 a. | Oxygen | c. | Hydrogen | b. | Copper | d. | Carbon |
|
|
|
6.
|
What is made from tin and lead?
a. | copper | c. | solder | b. | aluminum | d. | flux |
|
|
|
7.
|
Which of the following materials makes a good insulator?
a. | Silver | c. | Plastic | b. | Copper | d. | Water |
|
|
|
8.
|
Which of the following makes a good conductor?
a. | plastic | c. | wood | b. | rubber | d. | gold |
|
Yes/No
Indicate whether you
agree with the statement.
|
|
|
9.
|
1. Shown below are the electron orbital rings
for the elements Lead (Pd) and Tin (Sn). Complete the diagrams by labeling the number of
electrons that belong in each of the orbital rings.
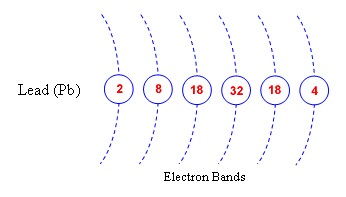
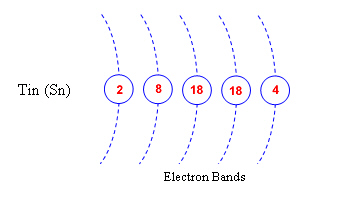
Based on their
electron configuration, would tin and lead be good conductors?
|
Matching
|
|
|
a. | semiconductors | c. | conductors | b. | insulators |
|
|
|
10.
|
Elements whose electrons are unstable.
|
|
|
11.
|
Elements whose electrons are stable.
|
|
|
12.
|
Elements that are not considered conductors or insulators.
|