Online Assessment
Instructions
1) Type in your first and last name in the
“Name” box in the top-left corner.
2) Next type in your teacher’s name in
the “ID” box.
3) Then type in your school’s full name in the
“Email” box.
4) Select the best answer for each question.
5) When you
are finished click the “Grade and Submit” button.
6) The grade will be emailed
to your teacher.
|
|
|
1.
|
NOTE: If your teacher’s last
name is Elliott, then you are doing the wrong assessment.
---This assessment is only for
students of other teachers.
---If your teacher is Mr. Elliott, then please go back to the
website and click on the first assessment link instead. Thanks!
The formula below represents a chemical
reaction.
AgNO3
(silver nitrate) +
NaCl (sodium chloride)à AgCl (silver
chloride) +NaNO3 (sodium nitrate)
Which substance is a product of this reaction?
a. | AgCl (silver
chloride) | c. | Na
(sodium) | b. | NaCl (sodium chloride) | d. | Ag (silver) |
|
|
|
2.
|
A chemical equation is shown
below.
2NaCl +2H2O àCl2 + H2 + 2NaOH
Which substance is a
reactant?
|
|
|
3.
|
When a rocket ship blasts off it is due to a
powerful chemical reaction as shown below.
10Al + 6NH4ClO4  4Al2O3 + 12H2O + 3N2
4Al2O3 + 12H2O + 3N2
Identify two
products in this reaction.
a. | Al (aluminum) & NH4
(ammonium) | c. | Cl (Chloride) and O (Oxygen) | b. | Al (aluminum) and O
(Oxygen) | d. | H2O (water) and N (nitrogen) |
|
|
|
4.
|
A chemical equation is shown
below.
2H2O ç 2H2 +
O2
Which substance is a
reactant?a. | H2O | c. | Dihydrogen
Monoxide | b. | Hydrogen | d. | Water |
|
|
|
5.
|
The Statue of Liberty is made of copper (Cu). This
metal changes to a light-green color when it reacts with oxygen (O) to form copper oxide (CuO).
| 4Cu + O2  2Cu2O
2Cu2O | |
Which chemical formula is the product
from the eqation shown above?
a. | Oxygen
(O) | b. | 2Cu2O | c. | Copper (Cu) | d. | O2 |
|
|
|
6.
|
A balanced chemical
equation is shown below.
2PbO + 4NO2 + O2
à 2Pb(NO3)
The product in
this equation is...?
a. | O2
| b. | NO2
| c. | PbO | d. | Pb(NO3)2 |
|
|
|
7.
|
Examine the following chemical
reaction
Iron (Fe) reacts with Oxygen (O) to form
iron oxide (FeO) which is commonly called rust.
2Fe + 3O2  2FeO3
2FeO3
Which chemical formula shown below
is the product of this reaction? a. | 2FeO3 | b. | Iron (Fe) | c. | 3O2 | d. | Iron Oxide |
|
|
|
8.
|
Soured milk is the result of a chemical reaction.
Below is the equation for this reaction.Which of the following choices is the
product of this chemical reaction?
a. | 4C3H6O3 | c. | H2O | b. | C12H22O11 | d. | CO2 |
|
|
|
9.
|
A chemical equation is shown
below.
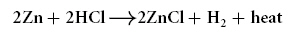 The element zinc (Zn) can best be described as
a...?a. | compound | b. | gas | c. | reactant | d. | mixture |
|
|
|
10.
|
A chemical equation is shown
below.
2H2O ç 2H2 +
O2
Which of the following choices is
the product of this chemical reaction?a. | Hydrogen | c. | Water | b. | H2 | d. | Oxygen |
|